Language & Cognition
Motor Development
Motor development occurs in an orderly sequence as infants move from reflexive reactions (e.g., sucking and rooting) to more advanced motor functioning. As mentioned during the prenatal section, development occurs according to the Cephalocaudal (from head to tail) and Proximodistal (from the midline outward) principles. For instance, babies first learn to hold their heads up, then to sit with assistance, then to sit unassisted, followed later by crawling, pulling up, cruising or walking while holding on to something, and then unassisted walking (Eisenberg, Murkoff, & Hathaway, 1989). As motor skills develop, there are certain developmental milestones that young children should achieve. For each milestone there is an average age, as well as a range of ages in which the milestone should be reached. An example of a developmental milestone is a baby holding up its head. Babies on average are able to hold up their heads at 6 weeks old, and 90% of babies achieve this between 3 weeks and 4 months old. On average, most babies sit alone at 7 months. Sitting involves both coordination and muscle strength, and 90% of babies achieve this milestone between 5 and 9 months old. If the child is displaying delays on several milestones, that is reason for concern, and the parent or caregiver should check in with the child’s pediatrician. Developmental delays can be identified and addressed through early intervention.

Motor Skills refer to our ability to move our bodies and manipulate objects. Gross motor skills focus on large muscle groups that control our head, torso, arms and legs and involve larger movements (e.g., balancing, running, and jumping). These skills begin to develop first. Examples include moving to bring the chin up when lying on the stomach, moving the chest up, and rocking back and forth on hands and knees. But it also includes exploring an object with one’s feet as many babies do as early as 8 weeks of age if seated in a carrier or other device that frees the hips. This may be easier than reaching for an object with the hands, which requires much more practice (Berk, 2007). Sometimes an infant will try to move toward an object while crawling and surprisingly move backward because of the greater amount of strength in the arms than in the legs.
Fine motor skills focus on the muscles in our fingers, toes, and eyes, and enable coordination of small actions (e.g., grasping a toy, writing with a pencil, and using a spoon). Newborns cannot grasp objects voluntarily but do wave their arms toward objects of interest. At about 4 months of age, the infant is able to reach for an object, first with both arms and within a few weeks, with only one arm. At this age grasping an object involves the use of the fingers and palm, but no thumbs. This is known as the Palmer Grasp. The use of the thumb comes at about 9 months of age when the infant is able to grasp an object using the forefinger and thumb. Now the infant uses a Pincer Grasp, and this ability greatly enhances the ability to control and manipulate an object. Infants take great delight in this newfound ability. They may spend hours picking up small objects from the floor and placing them in containers. By 9 months, an infant can also watch a moving object, reach for it as it approaches, and grab it.
Sensory Capacities
Throughout much of history, the newborn was considered a passive, disorganized being who possessed minimal abilities. William James, an early psychologist, had described the newborn’s world as “a blooming, buzzing confusion” (Shaffer, 1985). However, current research techniques have demonstrated just how developed the newborn is with especially organized sensory and perceptual abilities.
Vision. The womb is a dark environment void of visual stimulation. Consequently, vision is one of the most poorly developed senses at birth, and time is needed to build neural pathways between the eyes and the brain (American Optometric Association [AOA], 2019). Newborns typically cannot see further than 8 to 10 inches away from their faces (AOA, 2019). An 8-week old’s vision is 20/300. This means an object 20 feet away from an infant has the same clarity as an object 300 feet away from an adult with normal vision. By 3-months visual acuity has sharpened to 20/200, which would allow them the see the letter E at the top of a standard eye chart (Hamer, 2016). As a result, the world initially looks blurry to young infants (Johnson & deHaan, 2015).
Why is visual acuity so poor in the infant? The fovea, which is the central field of vision in the retina and allows us to see sharp detail, is not fully developed at birth, and does not start to reach adult levels of development until 15 months (Li & Ding, 2017). Even by 45 months some of the sensory neurons (cones) of the fovea are still not fully grown. Can babies see color? Young infants can perceive color, but the colors need to be very pure forms of basic colors, such as vivid red or green rather than weaker pastel shades. Most studies report that babies can see the full spectrum of colors by five months of age (AOA, 2019).
Newborn infants prefer and orient to face-like stimuli more than they do other patterned stimuli (Farroni et al., 2005). They also prefer images of faces that are upright and not scrambled (Chien, 2011). Infants also quickly learn to distinguish the face of their mother from faces of other women (Bartrip, Morton, & De Schonen, 2001). When viewing a person’s face, one-month olds fixate on the outer edges of the face rather than the eyes, nose, or mouth, but two-month olds gaze more at the inner features, especially the eyes (Hainline, 1978). Researchers have examined the development of attention and tracking in the visual system and have found the following for young infants:
- One-month-olds have difficulty disengaging their attention and can spend several minutes fixedly gazing at a stimulus (Johnson & deHaan, 2015).
- Aslin (1981) found that when tracking an object visually, the eye movements of newborns and one-month olds are not smooth but saccadic, that is step-like jerky movements. Aslin also found that eye movements lag behind an object’s motion. This means young infants do not anticipate the trajectory of the object. By two months of age, their eye movements are becoming smoother, but they still lag behind the motion of the object and will not achieve this until about three to four months of age (Johnson & deHaan, 2015).
- Newborns also orient more to the visual field toward the side of the head, than to the visual field on either side of the nose (Lewis, Maurer, & Milewski, 1979). By two to three months, stimuli in both fields are now attended to equally (Johnson & deHaan, 2015).
Binocular vision, which requires input from both eyes, is evident around the third month and continues to develop during the first six months (Atkinson & Braddick, 2003). By six months infants can perceive depth perception in pictures as well (Sen, Yonas, & Knill, 2001). Infants who have experience crawling and exploring will pay greater attention to visual cues of depth and modify their actions accordingly (Berk, 2007).

Hearing. The infant’s sense of hearing is very keen at birth, and the ability to hear is evidenced as soon as the seventh month of prenatal development. Newborns prefer their mother’s voices over another female even if speaking the same material (DeCasper & Fifer, 1980). Additionally, they will register in utero specific information heard from their mother’s voice. DeCasper and Spence (1986) tested 16 infants (average age of 55.8 hours) whose mothers had previously read to them prenatally. The mothers read several passages to their fetuses, including the first 28 paragraphs of the Cat in the Hat, beginning when they were 7 months pregnant. The fetuses had been exposed to the stories an average of 67 times or 3.5 hours. When the experimental infants were tested, the target stories (previously heard) were more reinforcing than the novel story as measured by their rate of sucking. However, for control infants, the target stories were not more reinforcing than the novel story indicating that the experimental infants had heard them before.
An infant can distinguish between very similar sounds as early as one month after birth and can distinguish between a familiar and non-familiar voice even earlier. Infants are especially sensitive to the frequencies of sounds in human speech and prefer the exaggeration of infant-directed speech, which will be discussed later. Additionally, infants are innately ready to respond to the sounds of any language, but between six and nine months they show preference for listening to their native language (Jusczyk, Cutler, & Redanz, 1993). Their ability to distinguish the sounds that are not in the language around them diminishes rapidly (Cheour-Luhtanen, et al., 1995).
Touch and pain. Immediately after birth, a newborn is sensitive to touch and temperature, and is also highly sensitive to pain, responding with crying and cardiovascular responses (Balaban & Reisenauer, 2013). Newborns who are circumcised, which is the surgical removal of the foreskin of the penis, without anesthesia experience pain as demonstrated by increased blood pressure, increased heart rate, decreased oxygen in the blood, and a surge of stress hormones (United States National Library of Medicine, 2016). Research has demonstrated that infants who were circumcised without anesthesia experienced more pain and fear during routine childhood vaccines. Fortunately, today many local pain killers are currently used during circumcision.

Taste and smell. Studies of taste and smell demonstrate that babies respond with different facial expressions, suggesting that certain preferences are innate. Newborns can distinguish between sour, bitter, sweet, and salty flavors and show a preference for sweet flavors. Newborns also prefer the smell of their mothers. An infant only 6 days old is significantly more likely to turn toward its own mother’s breast pad than to the breast pad of another baby’s mother (Porter, Makin, Davis, & Christensen, 1992), and within hours of birth an infant also shows a preference for the face of its own mother (Bushnell, 2001; Bushnell, Sai, & Mullin, 1989).
Intermodality. Infants seem to be born with the ability to perceive the world in an intermodal way; that is, through stimulation from more than one sensory modality. For example, infants who sucked on a pacifier with either a smooth or textured surface preferred to look at a corresponding (smooth or textured) visual model of the pacifier. By 4 months, infants can match lip movements with speech sounds and can match other audiovisual events. Sensory processes are certainly affected by the infant’s developing motor abilities (Hyvärinen, Walthes, Jacob, Nottingham Chapin, & Leonhardt, 2014). Reaching, crawling, and other actions allow the infant to see, touch, and organize his or her experiences in new ways.
How are infants tested. Habituation procedures, that is measuring decreased responsiveness to a stimulus after repeated presentations, have increasingly been used to evaluate infants in studies of the development of perceptual and memory skills. Phelps (2005) describes a habituation procedure used when measuring the rate of the sucking reflex. Researchers first measure the initial baseline rate of sucking to a pacifier equipped with transducers that measure muscle contractions. Next, an auditory stimulus is presented, such as a human voice uttering a speech sound such as “da.” The rate of sucking will typically increase with the new sound, but then decrease to baseline levels as “da” is repeatedly presented, showing habituation. If the sound “ma” was then presented, the rate of sucking would again increase, demonstrating that the infant can discriminate between these two stimuli.
Additionally, the speed or efficiency with which infants show habituation has been shown to predict outcomes in behaviors, such as language acquisition and verbal and nonverbal intelligence. Infants who show difficulty during habituation, or habituate at slower than normal rates, have been found to be at an increased risk for significant developmental delays. Infants with Down syndrome, teratogen-exposed infants, malnourished infants, and premature infants have all been studied. Researchers have found that at the age of 16 months, high-risk infants show rates of habituation comparable to newborn infants (Phelps, 2005).
Learning Objectives: Cognitive Development in Infancy and Toddlerhood
- Explain the Piagetian concepts of schema, assimilation, and accommodation.
- List and describe the six substages of sensorimotor intelligence.
- Describe the characteristics of infant memory.
- Describe components and developmental progression of language.
- Identify and compare the theories of language.
Piaget and the Sensorimotor Stage
Piaget believed that children, even infants, actively try to make sense of their environments. He viewed intelligence, not as knowledge or facts we acquire, but as the processes through which we adapt to our environment. He argued that differences between children and adults are not based on the fact that children know less than adults, but because they think in different ways the adults do. Piaget used the clinical method in which he closely observed individual children in great detail over long periods of time in their natural environment. From these observations he developed his theory of cognitive development, which posits four qualitatively different stages (Piaget, 1954).
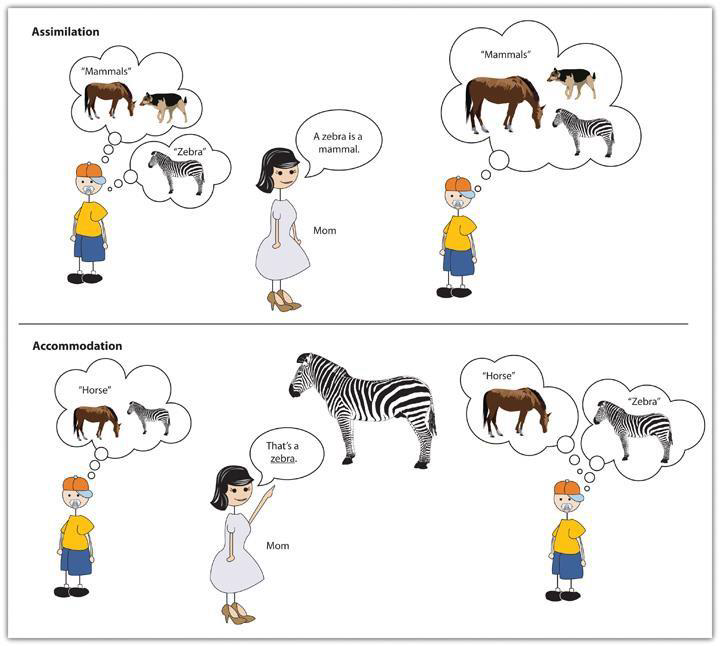
Schema, assimilation and accommodation. In addition to descriptions of different stages, Piaget was also very interested in the processes by which people come to understand the world (and in the process, to understand themselves). He was focused on universal physical properties of environments, like time, space, and causality, which he called logic-mathematical thought. He argued that people make sense of the world by interacting with it. He assumed that all people, even infants, are active, curious, energetic, and intrinsically motivated, and it is through their active attempts to “make things happen” that they learn about natural laws. Piaget held that, as they go, infants and children construct models of how the world works, which are partial, incomplete, and not totally correct. He called these models, schema, which an be thought of as frameworks for organizing information. As we continue interacting with the world, we keep trying out our mental models, and eventually encounter experiences that contradict them. These contradictions allow us to revise our models so that they can better account for the interactions we are experiencing. As models are revised, they are more adaptive– meaning that they guide our actions more effectively as we try to reach our goals.
Children develop their models, that is, their schemata, through processes of assimilation and accommodation. When faced with something new, a child may demonstrate assimilation, which is fitting the new information into an existing schema, such as calling all animals with four legs “doggies” because he or she has the concept of doggie. When it becomes clear that the new information no longer fits into the old schema, instead of assimilating the information, the child may demonstrate accommodation, which is expanding the framework of knowledge to accommodate the new situation and thus learning a new concept to more accurately name the animal. For example, recognizing that a horse is different than a zebra means the child has accommodated, and now the child has both a zebra schema and a horse schema. Even as adults we continue to try and “make sense” of new situations by determining whether they fit into our old way of thinking (assimilation) or whether we need to modify our thoughts (accommodation).
Piaget also described a process of organization, in which we combine existing schemes into new and more complex ones. By grouping and re-arranging schemas, and connecting them together, we can grow and refine our knowledge structures. Finally, he pointed to the process of disequilibration, where we detect discrepancies or contradictions between the models we are constructing and the experiences we are having in our interactions with the world. These contradictions can produce confusion or frustration, but they are developmentally helpful, because they lead to attempts to readjust actions and models so they are in better alignment, though a process called equilibration. Disequilibration and equilibration can also be applied to models themselves, as we detect that sub-parts of models contradict each other or are not in alignment. Though processes of equilibration we can re-organize models so that they are more internally consistent and coherent.
Cognitive development during infancy. Piaget’s theories revolutionized the way that developmentalists thought about infants. He was one of the first researchers to argue that infants are intelligent and they are busily constructing their own understandings of the world through their interactions with the environment. According to the Piagetian perspective, infants learn about the world primarily through their senses and motor abilities (Harris, 2005). These basic motor and sensory abilities provide the foundation for the cognitive skills that will emerge during the subsequent stages of cognitive development. The first stage of cognitive development is referred to as the sensorimotor stage and it occurs through six substages. Table 3.5 identifies the ages typically associated with each substage.
Table 3.5 Infant Ages for the Six Substages of the Sensorimotor Stage
| Substage 1 | Reflexes (0–1 month) |
| Substage 2 | Primary Circular Reactions (1–4 months) |
| Substage 3 | Secondary Circular Reactions (4–8 months) |
| Substage 4 | Coordination of Secondary Circular Reactions (8–12 months) |
| Substage 5 | Tertiary Circular Reactions (12–18 months) |
| Substage 6 | Beginning of Representational Thought (18–24 months) |
adapted from Lally & Valentine-French, 2019
Substage 1: Reflexes. Newborns learn about their world through the use of their reflexes, such as when sucking, reaching, and grasping. Eventually the use of these reflexes becomes more deliberate and purposeful.
Substage 2: Primary Circular Reactions. During these next 3 months, the infant begins to actively involve his or her own body in some form of repeated activity. An infant may accidentally engage in a behavior and find it interesting such as making a vocalization. This interest motivates the infant to try to do it again and helps the infant learn a new behavior that originally occurred by chance. The behavior is identified as circular because of the repetition, and as primary because it centers on the infant’s own body.

Substage 3: Secondary Circular Reactions. The infant begins to interact with objects in the environment. At first the infant interacts with objects (e.g., a crib mobile) accidentally, but then these contacts with the objects are deliberate and become a repeated activity. The infant becomes more and more actively engaged in the outside world and takes delight in being able to make things happen. Repeated motion brings particular interest as, for example, the infant is able to bang two lids together from the cupboard when seated on the kitchen floor.
Substage 4: Coordination of Secondary Circular Reactions. The infant combines these basic reflexes and simple behaviors and uses planning and coordination to achieve a specific goal. Now the infant can engage in behaviors that others perform and anticipate upcoming events. Perhaps because of continued maturation of the prefrontal cortex, the infant become capable of having a thought and carrying out a planned, goal-directed activity. For example, an infant sees a toy car under the kitchen table and then crawls, reaches, and grabs the toy. The infant is coordinating both internal and external activities to achieve a planned goal.
Substage 5: Tertiary Circular Reactions. The toddler is considered a “little scientist” and begins exploring the world in a trial-and-error manner, using both motor skills and planning abilities. For example, the child might throw her ball down the stairs to see what happens. The toddler’s active engagement in experimentation helps them learn about their world.

Substage 6: Beginning of Representational Thought. The sensorimotor period ends with the appearance of symbolic or representational thought. The toddler now has a basic understanding that objects can be used as symbols. Additionally, the child is able to solve problems using mental strategies, to remember something heard days before and repeat it, and to engage in pretend play. This initial movement from a “hands-on” approach to knowing about the world to the more mental world of substage six marks the transition to preoperational thought.
Development of object permanence. A critical milestone during the sensorimotor period is the development of object permanence. Object permanence is the understanding that even if something is out of sight, it still exists (Bogartz, Shinskey, & Schilling, 2000). According to Piaget, young infants cannot represent objects mentally, so they do not remember it after it has been removed from sight. Piaget studied infants’ reactions when a toy was first shown to them and then hidden under a blanket. Infants who had already developed object permanence would reach for the hidden toy, indicating that they knew it still existed, whereas infants who had not developed object permanence would appear confused. Piaget emphasizes this construct because it is an objective way for children to demonstrate how they mentally represent their world. Children have typically acquired this milestone by 8 months. Once toddlers have mastered object permanence, they enjoy games like hide and seek, and they realize that when someone leaves the room they are still in the world. Toddlers also point to pictures in books and look in appropriate places when you ask them to find objects.
In Piaget’s view, around the same time children develop object permanence, they also begin to exhibit stranger anxiety, which is a fear of unfamiliar people (Crain, 2005). Babies may demonstrate this by crying and turning away from a stranger, by clinging to a caregiver, or by attempting to reach their arms toward familiar faces, such as parents. Stranger anxiety results when a child is unable to assimilate the stranger into an existing schema; therefore, she cannot predict what her experience with that stranger will be like, which results in a fear response.
Critique of Piaget. Piaget thought that children’s ability to understand objects, such as learning that a rattle makes a noise when shaken, was a cognitive skill that develops slowly as a child matures and interacts with the environment. Today, developmental psychologists question the timetables Piaget laid out. Researchers have found that even very young children understand objects and how they work long before they have experience with those objects (Baillargeon, 1987; Baillargeon, Li, Gertner, & Wu, 2011). For example, Piaget believed that infants did not fully master object permanence until substage 5 of the sensorimotor period (Thomas, 1979). However, infants seem to be able to recognize that objects have permanence at much younger ages. Diamond (1985) found that infants show earlier knowledge if the waiting period is shorter. At age 6 months, they retrieved the hidden object if their wait for retrieving the object is no longer than 2 seconds, and at 7 months if the wait is no longer than 4 seconds.
Development of Memory during Infancy
Memory requires the capacity to mentally represent experience, so it should not be surprising that infant memory is rather fleeting and fragile. As a result, older children and adults experience infantile amnesia, the inability to recall memories from the first few years of life. Several hypotheses have been proposed for this amnesia. From the biological perspective, it has been suggested that infantile amnesia is due to the immaturity of the infant brain, especially those areas that are crucial to the formation of autobiographical memory, such as the hippocampus. From the cognitive perspective, it has been suggested that the lack of linguistic skills of babies and toddlers limit their ability to mentally represent events; thereby, reducing their ability to encode memory. Moreover, even if infants do form such early memories, older children and adults may not be able to access them because they may be employing very different, more linguistically based, retrieval cues than infants used when forming largely photographic or visual memories. Finally, social theorists argue that episodic memories of personal experiences may hinge on an understanding of “self”, something that is clearly lacking in infants and young toddlers.
However, in a series of clever studies Carolyn Rovee-Collier and her colleagues have demonstrated that infants can remember events from their life, even if these memories are short-lived. Three-month-old infants were taught that they could make a mobile hung over their crib shake by kicking their legs. The infants were placed in their crib, on their backs. A ribbon was tied to one foot and the other end to a mobile. At first infants made random movements, but then came to realize that by kicking they could make the mobile shake. After two 9-minute sessions with the mobile, the mobile was removed. One week later the mobile was reintroduced to one group of infants and most of the babies immediately started kicking their legs, indicating that they remembered their prior experience with the mobile. A second group of infants was shown the mobile two weeks later, and the babies made only random movements. The memory had faded (Rovee-Collier, 1987; Giles & Rovee-Collier, 2011). Rovee-Collier and Hayne (1987) found that 3-month-olds could remember the mobile after two weeks if they were shown the mobile and watched it move, even though they were not tied to it. This reminder helped most infants to remember the connection between their kicking and the movement of the mobile. Like many researchers of infant memory, Rovee-Collier (1990) found infant memory to be very context dependent. In other words, the sessions with the mobile and the later retrieval sessions had to be conducted under very similar circumstances or else the babies would not remember their prior experiences with the mobile. For instance, if the first mobile had had yellow blocks with blue letters, but at the later retrieval session the blocks were blue with yellow letters, the babies would not kick.
Infants older than 6 months of age can retain information for longer periods of time; they also need less reminding to retrieve information in memory. Studies of deferred imitation, that is, the imitation of actions after a time delay, can occur as early as six-months of age (Campanella & Rovee-Collier, 2005), but only if infants are allowed to practice the behavior they were shown. By 12 months of age, infants no longer need to practice the behavior in order to retain the memory for four weeks (Klein & Meltzoff, 1999).
Language Development
Our vast intelligence also allows us to have language, a system of communication that uses symbols in a regular way to create meaning. Language gives us the ability to communicate our thoughts to others by talking, reading, and writing. Although other species have at least some ability to communicate, as far as we know, none of them have language. There are many components of language that will now be reviewed.
Components of Language
Phoneme: A phoneme is the smallest unit of sound that makes a meaningful difference in a language. The word “bit” has three phonemes. In spoken languages, phonemes are produced by the positions and movements of the vocal tract, including our lips, teeth, tongue, vocal cords, and throat, whereas in sign languages phonemes are defined by the shapes and movement of the hands.
There are hundreds of unique phonemes that can be made by human speakers, but most languages only use a small subset of the possibilities. English contains about 45 phonemes, whereas other languages have as few as 15 and others more than 60. The Hawaiian language contains fewer phonemes as it includes only 5 vowels (a, e, i, o, and u) and 7 consonants (h, k, l, m, n, p, and w).
Infants are born able to detect all phonemes, but they lose their ability to do so as they get older; by 10 months of age a child’s ability to recognize phonemes becomes very similar to that of the adult speakers of the native language. Phonemes that were initially differentiated come to be treated as equivalent (Werker & Tees, 2002).
Morpheme: Whereas phonemes are the smallest units of sound in language, a morpheme is a string of one or more phonemes that makes up the smallest units of meaning in a language. Some morphemes are prefixes and suffixes used to modify other words. For example, the syllable “re-” as in “rewrite” or “repay” means “to do again,” and the suffix “-est” as in “happiest” or “coolest” means “to the maximum.”
Semantics: Semantics refers to the set of rules we use to obtain meaning from morphemes. For example, adding “ed” to the end of a verb makes it past tense.
Syntax: Syntax is the set of rules of a language by which we construct sentences. Each language has a different syntax. The syntax of the English language requires that each sentence have a noun and a verb, each of which may be modified by adjectives and adverbs. Some syntaxes make use of the order in which words appear. For example, in English the meaning of the sentence “The man bites the dog” is different from “The dog bites the man.”
Pragmatics: The social side of language is expressed through pragmatics, or how we communicate effectively and appropriately with others. Examples of pragmatics include turn-taking, staying on topic, volume and tone of voice, and appropriate eye contact.
Lastly, words do not possess fixed meanings, but change their interpretation as a function of the context in which they are spoken. We use contextual information, the information surrounding language, to help us interpret it. Examples of contextual information include our knowledge and nonverbal expressions, such as facial expressions, postures, and gestures. Misunderstandings can easily arise if people are not attentive to contextual information or if some of it is missing, such as it may be in newspaper headlines or in text messages.
Language Developmental Progression
An important aspect of cognitive development is language acquisition. The order in which children learn language structures is consistent across children and cultures (Hatch, 1983). Starting before birth, babies begin to develop language and communication skills. At birth, babies recognize their mother’s voice and can discriminate between the language(s) spoken by their mothers and foreign languages, and they show preferences for faces that are moving in synchrony with audible language (Blossom & Morgan, 2006; Pickens et al., 1994; Spelke & Cortelyou, 1981).

Do newborns communicate? Of course, they do. They do not, however, communicate with the use of oral language. Instead, they communicate their thoughts and needs with body posture (being relaxed or still), gestures, cries, and facial expressions. A person who spends adequate time with an infant can learn which cries indicate pain and which ones indicate hunger, discomfort, or frustration.
Intentional vocalizations. In terms of producing spoken language, babies begin to coo almost immediately. Cooing is a one-syllable combination of a consonant and a vowel sound (e.g., coo or ba). Interestingly, babies replicate sounds from their own languages. A baby whose parents speak French will coo in a different tone than a baby whose parents speak Spanish or Urdu. These gurgling, musical vocalizations can serve as a source of entertainment to an infant who has been laid down for a nap or seated in a carrier on a car ride. Cooing serves as practice for vocalization, as well as the infant hears the sound of his or her own voice and tries to repeat sounds that are entertaining. Infants also begin to learn the pace and pause of conversation as they alternate their vocalization with that of someone else and then take their turn again when the other person’s vocalization has stopped.
At about four to six months of age, infants begin making even more elaborate vocalizations that include the sounds required for any language. Guttural sounds, clicks, consonants, and vowel sounds stand ready to equip the child with the ability to repeat whatever sounds are characteristic of the language heard. Eventually, these sounds will no longer be used as the infant grows more accustomed to a particular language.
At about 7 months, infants begin babbling, engaging in intentional vocalizations that lack specific meaning and comprise a consonant-vowel repeated sequence, such as ma-ma-ma, da-da-da. Children babble as practice in creating specific sounds, and by the time they are a 1 year old, the babbling uses primarily the sounds of the language that they are learning (de Boysson-Bardies, Sagart, & Durand, 1984). These vocalizations have a conversational tone that sounds meaningful even though it is not. Babbling also helps children understand the social, communicative function of language. Children who are exposed to sign language babble in sign by making hand movements that represent real language (Petitto & Marentette, 1991).
Gesturing. Children communicate information through gesturing long before they speak, and there is some evidence that gesture usage predicts subsequent language development (Iverson & Goldin-Meadow, 2005). Deaf babies also use gestures to communicate wants, reactions, and feelings. Because gesturing seems to be easier than vocalization for some toddlers, sign language is sometimes taught to enhance an infant’s ability to communicate by making use of the ease of gesturing. The rhythm and pattern of language is used when deaf babies sign, just as it is when hearing babies babble.
Understanding. At around ten months of age, the infant can understand more than he or she can say, which is referred to as receptive language. You may have experienced this phenomenon as well if you have ever tried to learn a second language. You may have been able to follow a conversation more easily than contribute to it. One of the first words that children understand is their own name, usually by about 6 months, followed by commonly used words like “bottle,” “mama,” and “doggie” by 10 to 12 months (Mandel, Jusczyk, & Pisoni, 1995). Infants shake their head “no” around 6–9 months, and they respond to verbal requests to do things like “wave bye-bye” or “blow a kiss” around 9–12 months. Children also use contextual information, particularly the cues that parents provide, to help them learn language. Children learn that people are usually referring to things that they are looking at when they are speaking (Baldwin, 1993), and that that the speaker’s emotional expressions are related to the content of their speech.
Holophrasic speech. Children begin using their first words at about 12 or 13 months of age and may use partial words to convey thoughts at even younger ages. These one-word expressions are referred to as holophrasic speech. For example, the child may say “ju” for the word “juice” and use this sound when referring to a bottle. The listener must interpret the meaning of the holophrase, and when this is someone who has spent time with the child, interpretation is not too difficult. But someone who has not been around the child will have trouble knowing what is meant. Imagine the parent who to a friend exclaims, “Ezra’s talking all the time now!” The friend hears only “ju ga da” to which the parent explains means, “I want some milk when I go with Daddy.”
Language Errors: The early utterances of children contain many errors, for instance, confusing /b/ and /d/, or /c/ and /z/. The words children create are often simplified, in part because they are not yet able to make the more complex sounds of the real language (Dobrich & Scarborough, 1992). Children may say “keekee” for kitty, “nana” for banana, and “vesketti” for spaghetti because it is easier. Often these early words are accompanied by gestures that may also be easier to produce than the words themselves. Children’s pronunciations become increasingly accurate between 1 and 3 years, but some problems may persist until school age.
A child who learns that a word stands for an object may initially think that the word can be used for only that particular object, which is referred to as underextension. Only the family’s Irish Setter is a “doggie”, for example. More often, however, a child may think that a label applies to all objects that are similar to the original object, which is called overextension. For example, all animals become “doggies”. The first error is often the result of children learning the meaning of a word in a specific context, while the second language error is a function of the child’s smaller vocabulary.
First words and cultural influences. If the child is using English, first words tend to be nouns. The child labels objects such as cup, ball, or other items that they regularly interact with. In a verb-friendly language such as Chinese, however, children may learn more verbs. This may also be due to the different emphasis given to objects based on culture. Chinese children may be taught to notice action and relationships between objects, while children from the United States may be taught to name an object and its qualities (color, texture, size, etc.). These differences can be seen when comparing interpretations of art by older students from China and the United States (Imai et al., 2008).
Two-word sentences and telegraphic (text message) speech. By the time they become toddlers, children have a vocabulary of about 50-200 words and begin putting those words together in telegraphic speech, such as “baby bye-bye” or “doggie pretty”. Words needed to convey messages are spoken, but the articles and other parts of speech necessary for grammatical correctness are not yet used. These expressions sound like a telegraph, or perhaps a better analogy today would be that they read like a text message. Telegraphic speech/text message speech occurs when unnecessary words are not used. “Give baby ball” is used rather than “Give the baby the ball.”
Infant-directed speech. Why is a horse a “horsie”? Have you ever wondered why adults tend to use “baby talk” or that sing-song type of intonation and exaggeration used when talking to children? This represents a universal tendency and is known as infant-directed speech. It involves exaggerating the vowel and consonant sounds, using a high-pitched voice, and delivering the phrase with great facial expression (Clark, 2009). Why is this done? Infants are frequently more attuned to the tone of voice of the person speaking than to the content of the words themselves and are aware of the target of speech. Werker, Pegg, and McLeod (1994) found that infants listened longer to a woman who was speaking to a baby than to a woman who was speaking to another adult. Adults may use this form of speech in order to clearly articulate the sounds of a word so that the child can hear the sounds involved. It may also be because when this type of speech is used, the infant pays more attention to the speaker and this sets up a pattern of interaction in which the speaker and listener are in tune with one another.
Theories of Language Development
Psychological theories of language learning differ in terms of the importance they place on nature and nurture. Remember that we are a product of both nature and nurture. Researchers now believe that language acquisition is partially inborn and partially learned through our interactions with our linguistic environment (Gleitman & Newport, 1995; Stork & Widdowson, 1974). First to be discussed are the biological theories, including nativist, brain areas and critical periods. Next, learning theory and social pragmatics will be presented.

Nativism. The linguist Noam Chomsky is a believer in the nature approach to language, arguing that human brains contain a language acquisition device (LAD) that includes a universal grammar that underlies all human language (Chomsky, 1965, 1972). According to this approach, each of the many languages spoken around the world (there are between 6,000 and 8,000) is an individual example of the same underlying set of procedures that are hardwired into human brains. Chomsky’s account proposes that children are born with a knowledge of general rules of syntax that determine how sentences are constructed. Language develops as long as the infant is exposed to it. No teaching, training, or reinforcement is required for language to develop as proposed by Skinner.
Chomsky differentiates between the deep structure of an idea; that is, how the idea is represented in the fundamental universal grammar that is common to all languages, and the surface structure of the idea or how it is expressed in any one language. Once we hear or express a thought in surface structure, we generally forget exactly how it happened. At the end of a lecture, you will remember a lot of the deep structure (i.e., the ideas expressed by the instructor), but you cannot reproduce the surface structure (the exact words that the instructor used to communicate the ideas).
Although there is general agreement among psychologists that babies are genetically programmed to learn language, there is still debate about Chomsky’s idea that there is a universal grammar that can account for all language learning. Evans and Levinson (2009) surveyed the world’s languages and found that none of the presumed underlying features of the language acquisition device were entirely universal. In their search they found languages that did not have noun or verb phrases, that did not have tenses (e.g., past, present, future), and even some that did not have nouns or verbs at all, even though a basic assumption of a universal grammar is that all languages should share these features.
Brain areas for language. For the 90% of people who are right-handed, language is stored and controlled by the left cerebral cortex, although for some left-handers this pattern is reversed. These differences can easily be seen in the results of neuroimaging studies that show that listening to and producing language creates greater activity in the left hemisphere than in the right. Broca’s area, an area in front of the left hemisphere near the motor cortex, is responsible for language production (Figure 3.28). This area was first localized in the 1860s by the French physician Paul Broca, who studied patients with lesions to various parts of the brain. Wernicke’s area, an area of the brain next to the auditory cortex, is responsible for language comprehension.
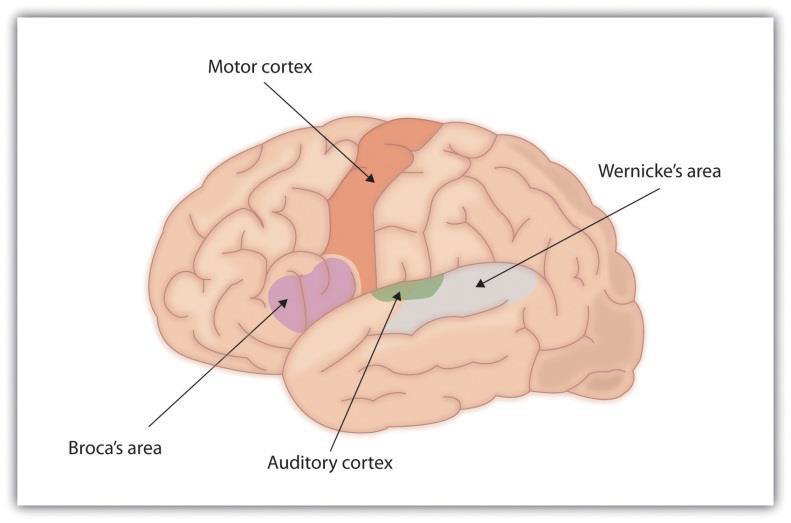
For most people the left hemisphere is specialized for language. Broca’s area, near the motor cortex, is involved in language production, whereas Wernicke’s area, near the auditory cortex, is specialized for language comprehension.

Is there a critical period for learning language? Psychologists believe there is a critical period, a time in which learning can easily occur, for language. This critical period appears to be between infancy and puberty (Lenneberg, 1967; Penfield & Roberts, 1959), but isolating the exact timeline has been elusive. Children who are not exposed to language early in their lives will likely never grasp the grammatical and communication nuances of language. Case studies, including Victor the “Wild Child,” who was abandoned as a baby in 18th century France and not discovered until he was 12, and Genie, a child whose parents kept her locked away from 18 months until 13 years of age, are two examples of children who were deprived of language. Both children made some progress in socialization after they were rescued, but neither of them ever developed a working understanding of language (Rymer, 1993). Yet, such case studies are fraught with many confounds. How much did the years of social isolation and malnutrition contribute to their problems in language development?
A better test for the notion of critical periods for language is found in studies of children with hearing loss. Several studies show that the earlier children are diagnosed with hearing impairment and receive treatment, the better the child’s long-term language development. For instance, Stika et al. (2015) reported that when children’s hearing loss was identified during newborn screening, and subsequently addressed, the majority showed normal language development when later tested at 12-18 months. Fitzpatrick, Crawford, Ni, and Durieux-Smith (2011) reported that early language intervention in children who were moderately to severely hard of hearing, demonstrated normal outcomes in language proficiency by 4 to 5 years of age. Tomblin et al. (2015) reported that children who were fit with hearing aids by 6 months of age showed good levels of language development by age 2. Those whose hearing was not corrected until after 18 months showed lower language performance, even in the early preschool years. However, this study did reveal that those whose hearing was corrected by toddlerhood had greatly improved language skills by age 6. The research with hearing impaired children reveals that this critical period for language development is not exclusive to infancy, and that the brain is still receptive to language development in early childhood. Fortunately, it is has become routine to screen hearing in newborns, because when hearing loss is not treated early, it can delay spoken language, literacy, and impact children’s social skills (Moeller & Tomblin, 2015).

Learning theory. Perhaps the most straightforward explanation of language development is that it occurs through the principles of learning, including association and reinforcement (Skinner, 1953). Additionally, Bandura (1977) described the importance of observation and imitation of others in learning language. There must be at least some truth to the idea that language is learned through environmental interactions or nurture. Children learn the language that they hear spoken around them rather than some other language. Also supporting this idea is the gradual improvement in language skills over time. It seems that children modify their language through imitation and reinforcement, such as parental praise and being understood. For example, when a two-year-old child asks for juice, he might say, “me juice,” to which his mother might respond by giving him a cup of apple juice. However, language cannot be entirely learned. For one, children learn words too fast for them to be learned through reinforcement. Between the ages of 18 months and 5 years, children learn up to 10 new words every day (Anglin, 1993). More importantly, language is more generative than it is imitative. Language is not a predefined set of ideas and sentences that we choose when we need them, but rather a system of rules and procedures that allows us to create an infinite number of statements, thoughts, and ideas, including those that have never previously occurred. When a child says that she “swimmed” in the pool, for instance, she is showing generativity. No adult speaker of English would ever say “swimmed,” yet it is easily generated from the normal system of producing language.

Other evidence that refutes the idea that all language is learned through experience comes from the observation that children may learn languages better than they ever hear them. Deaf children whose parents do not communicate using ASL very well nevertheless are able to learn it perfectly on their own and may even make up their own language if they need to (Goldin-Meadow & Mylander, 1998). A group of deaf children in a school in Nicaragua, whose teachers could not sign, invented a way to communicate through made-up signs (Senghas, Senghas, & Pyers, 2005). The development of this new Nicaraguan Sign Language has continued and changed as new generations of students have come to the school and started using the language. Although the original system was not a real language, it is becoming closer and closer every year, showing the development of a new language in modern times.
Social pragmatics. Another view emphasizes the very social nature of human language. Language from this view is not only a cognitive skill, but also a social one. Language is a tool humans use to communicate, connect to, influence, and inform others. Most of all, language comes out of a need to cooperate. The social nature of language has been demonstrated by a number of studies showing that children use several pre-linguistic skills (such as pointing and other gestures) to communicate not only their own needs, but what others may need. So, a child watching her mother search for an object may point to the object to help her mother find it. Eighteen-month to 30-month-olds have been shown to make linguistic repairs when it is clear that another person does not understand them (Grosse, Behne, Carpenter & Tomasello, 2010). Grosse et al. (2010) found that even when the child was given the desired object, if there had been any misunderstanding along the way (such as a delay in being handed the object, or the experimenter calling the object by the wrong name), children will make linguistic repairs. This would suggest that children are using language not only as a means of achieving some material goal, but also to make themselves understood in the mind of another person.
Supplemental Materials
Planful Problem-solving during Late Infancy
To give you a sense of what infants’ cognitive capacities allow them to do, here are some video clips of planful problem-solving at 8 months and about a year old:
How do we both get on the bed?
Optional Reading: The Brain in the First Two Years
Some of the most dramatic physical change that occurs during this period is in the brain. We are born with most of the brain cells that we will ever have; that is, about 85 billion neurons whose function is to store and transmit information (Huttenlocher & Dabholkar, 1997). While most of the brain’s neurons are present at birth, they are not fully mature. During the next several years dendrites, or branching extensions that collect information from other neurons, will undergo a period of exuberance. Because of this proliferation of dendrites, by age two a single neuron might have thousands of dendrites. Synaptogenesis, or the formation of connections between neurons, continues from the prenatal period forming thousands of new connections during infancy and toddlerhood. This period of rapid neural growth is referred to as synaptic blooming.
The blooming period of neural growth is then followed by a period of synaptic pruning, where neural connections are reduced thereby making those that are used much stronger. It is thought that pruning causes the brain to function more efficiently, allowing for mastery of more complex skills (Kolb & Whishaw, 2011). Experience will shape which of these connections are maintained and which of these are lost. Ultimately, about 40 percent of these connections will be lost (Webb, Monk, and Nelson, 2001). Blooming occurs during the first few years of life, and pruning continues through childhood and into adolescence in various areas of the brain.
Another major change occurring in the central nervous system is the development of myelin, a coating of fatty tissues around the axon of the neuron (Carlson, 2014). Myelin helps insulate the nerve cell and speed the rate of transmission of impulses from one cell to another. This enhances the building of neural pathways and improves coordination and control of movement and thought processes. The development of myelin continues into adolescence but is most dramatic during the first several years of life.
The infant brain grows very fast. At birth the brain is about 250 grams (half a pound) and by one year it is already 750 grams (Eliot, 1999). Comparing to adult size, the newborn brain is approximately 33% of adult size at birth, and in just 90 days, it is already at 55% of adult size (Holland et al., 2014). Most of the neural activity is occurring in the cortex or the thin outer covering of the brain involved in voluntary activity and thinking. The cortex is divided into two hemispheres, and each hemisphere is divided into four lobes, each separated by folds known as fissures. If we look at the cortex starting at the front of the brain and moving over the top (see Figure 3.3), we see first the frontal lobe (behind the forehead), which is responsible primarily for thinking, planning, memory, and judgment. Following the frontal lobe is the parietal lobe, which extends from the middle to the back of the skull and which is responsible primarily for processing information about touch. Next is the occipital lobe, at the very back of the skull, which processes visual information. Finally, in front of the occipital lobe, between the ears, is the temporal lobe, which is responsible for hearing and language (Jarrett, 2015).
Although the brain grows rapidly during infancy, specific brain regions do not mature at the same rate. Primary motor areas develop earlier than primary sensory areas, and the prefrontal cortex, that is located behind the forehead, is the least developed (Giedd, 2015). As the prefrontal cortex matures, the child is increasingly able to regulate or control emotions, to plan activities, strategize, and have better judgment. This is not fully accomplished in infancy and toddlerhood, but continues throughout childhood, adolescence and into adulthood.
Lateralization is the process in which different functions become localized primarily on one side of the brain. For example, in most adults the left hemisphere is more active than the right during language production, while the reverse pattern is observed during tasks involving visuospatial abilities (Springer & Deutsch, 1993). This process develops over time, however, structural asymmetries between the hemispheres have been reported even in fetuses (Chi, Dooling, & Gilles, 1997; Kasprian et al., 2011) and infants (Dubois et al., 2009).
Lastly, neuroplasticity refers to the brain’s ability to change, both physically and chemically, to enhance its adaptability to environmental change and compensate for injury. The control of some specific bodily functions, such as movement, vision, and hearing, is performed in specified areas of the cortex, and if these areas are damaged, the individual will likely lose the ability to perform the corresponding function. The brain’s neurons have a remarkable capacity to reorganize and extend themselves to carry out these particular functions in response to the needs of the organism, and to repair any damage. As a result, the brain constantly creates new neural communication routes and rewires existing ones. Both environmental experiences, such as stimulation and events within a person’s body, such as hormones and genes, affect the brain’s plasticity. So too does age. Adult brains demonstrate neuroplasticity, but they are influenced less extensively than those of infants (Kolb & Fantie, 1989; Kolb & Whishaw, 2011).
References
American Optometric Association. (2019). Infant vision: Birth to 24 months of age. Retrieved from https://www.aoa.org/patients-and-public/good-vision-throughout-life/childrens-vision/infant-vision-birth-to-24-months-of-age
Anglin, J. M. (1993). Vocabulary development: A morphological analysis. Monographs of the Society for Research in Child Development, 58 10), v–165.
Atkinson, J., & Braddick, O. (2003). Neurobiological models of normal and abnormal visual development. In M. de Haan & M. H. Johnson (Eds.), The cognitive neuroscience of development (pp. 43– 71). Hove: Psychology Press.
Aslin, R. N. (1981). Development of smooth pursuit in human infants. In D. F. Fisher, R. A. Monty, & J. W. Senders (Eds.), Eye movements: Cognition and visual perception (pp. 31– 51). Hillsdale, NJ: Erlbaum.
Baillargeon, R. (1987. Object permanence in 3 ½ and 4 ½ year-old infants. Developmental Psychology, 22, 655-664.
Baillargeon, R., Li, J., Gertner, Y, & Wu, D. (2011). How do infants reason about physical events? In U. Goswami (Ed.), The Wiley-Blackwell handbook of childhood cognitive development. MA: John Wiley.
Balaban, M. T. & Reisenauer, C. D. (2013). Sensory development. In N. J. Salkind (Ed.), Encyclopedia of human development (pp. 1144-1147). New, York: Sage Publications.
Baldwin, D. A. (1993). Early referential understanding: Infants’ ability to recognize referential acts for what they are. Developmental Psychology, 29(5), 832–843.
Bandura, A. (1977). Social learning theory. Englewood Cliffs, NJ: Prentice Hall.
Bartrip J, Morton J, & De Schonen S. (2001). Responses to mother’s face in 3-week to 5-month-old infants. British Journal of Developmental Psychology, 19, 219–232
Berk, L. E. (2007). Development through the life span (4th ed.). Boston: Allyn and Bacon.
Blossom, M., & Morgan, J. L. (2006). Does the face say what the mouth says? A study of infants’ sensitivity to visual prosody. In 30th annual Boston University conference on language development, Somerville, MA.
Bushnell, I. W. R. (2001) Mother’s face recognition in newborn infants: Learning and memory. Infant Child Development, 10, 67-94.
Bushnell, I. W. R., Sai, F., Mullin, J. T. (1989). Neonatal recognition of mother’s face. British Journal of Developmental Psychology, 7, 3-15.
Campanella, J., & Rovee-Collier, C. (2005). Latent learning and deferred imitation at 3 months. Infancy, 7(3), 243-262.
Cheour-Luhtanen, M., Alho. K., Kujala, T., Reinikainen, K., Renlund, M., Aaltonen, O., … & Näätänen R. (1995). Mismatch negativity indicates vowel discrimination in newborns. Hearing Research, 82, 53–58.
Chien S. (2011). No more top-heavy bias: Infants and adults prefer upright faces but not top-heavy geometric or face-like patterns. Journal of Vision, 11(6):1–14.
Chomsky, N. (1965). Aspects of the theory of syntax. Cambridge, MA: MIT Press.
Chomsky, N. (1972). Language and mind. NY: Harcourt Brace.
Clark, E. V. (2009). What shapes children’s language? Child-directed speech and the process of acquisition. In V. C. M. Gathercole (Ed.), Routes to language: Essays in honor of Melissa Bowerman. NY: Psychology Press.
Crain, W. (2005). Theories of development concepts and applications (5th ed.). NJ: Pearson.
de Boysson-Bardies, B., Sagart, L., & Durand, C. (1984). Discernible differences in the babbling of infants according to target language. Journal of Child Language, 11(1), 1–15.
DeCasper, A. J., & Fifer, W. P. (1980). Of human bonding: Newborns prefer their mother’s voices. Science, 208, 1174-1176.
DeCasper, A. J., & Spence, M. J. (1986). Prenatal maternal speech influences newborns’ perception of speech sounds. Infant Behavior and Development, 9, 133-150.
Diamond, A. (1985). Development of the ability to use recall to guide actions, as indicated by infants’ performance on AB. Child Development, 56, 868-883.
Dobrich, W., & Scarborough, H. S. (1992). Phonological characteristics of words young children try to say. Journal of Child Language, 19(3), 597–616.
Eisenberg, A., Murkoff, H. E., & Hathaway, S. E. (1989). What to expect the first year. New York: Workman Publishing.
Evans, N., & Levinson, S. C. (2009). The myth of language universals: Language diversity and its importance for cognitive science. Behavioral and Brain Sciences, 32(5), 429–448.
Farroni, T., Johnson, M.H. Menon, E., Zulian, L. Faraguna, D., Csibra, G. (2005). Newborns’ preference for face-relevant stimuli: Effects of contrast polarity. Proceedings of the National Academy of Sciences of the United States of America, 102(47), 17245-17250.
Fitzpatrick, E.M., Crawford, L., Ni, A., & Durieux-Smith, A. (2011). A descriptive analysis of language and speech skills in 4-to-5-yr-old children with hearing loss. Ear and Hearing, 32(2), 605-616.
Giles, A., & Rovee-Collier, C. (2011). Infant long-term memory for associations formed during mere exposure. Infant Behavior and Development, 34 (2), 327-338.
Gleitman, L. R., & Newport, E. L. (1995). The invention of language by children: Environmental and biological influences on the acquisition of language. An Invitation to Cognitive Science, 1, 1-24.
Goldin-Meadow, S., & Mylander, C. (1998). Spontaneous sign systems created by deaf children in two cultures. Nature, 391(6664), 279–281.
Grosse, G., Behne, T., Carpenter, M., & Tomasello, M. (2010). Infants communicate in order to be understood. Developmental Psychology, 46(6), 1710-1722.
Hainline L. (1978). Developmental changes in visual scanning of face and nonface patterns by infants. Journal of Experimental Child Psychology, 25, 90–115.
Hamer, R. (2016). The visual world of infants. Scientific American, 104, 98-101.
Hatch, E. M. (1983). Psycholinguistics: A second language perspective. Rowley, MA: Newbury House Publishers.
Hyvärinen, L., Walthes, R., Jacob, N., Nottingham Chaplin, K., & Leonhardt, M. (2014). Current understanding of what infants see. Current Opthalmological Report, 2, 142-149. doi:10.1007/s40135-014-0056-2.
Imai, M., Li, L., Haryu, E., Hirsh-Pasek, K., Golinkoff, R. M., & Shigematsu, J. (2008). Novel noun and verb learning in Chinese, English, and Japanese children: Universality and language-specificity in novel noun and verb learning. Child Development, 79, 979-1000.
Iverson, J. M., & Goldin-Meadow, S. (2005). Gesture paves the way for language development. Psychological science, 16(5), 367-371.
Johnson, M. H., & deHaan, M. (2015). Developmental cognitive neuroscience: An introduction. Chichester, West Sussex: UK, Wiley & Sons.
Jusczyk, P.W., Cutler, A., & Redanz, N.J. (1993). Infants’ preference for the predominant stress patterns of English words. Child Development, 64, 675–687.
Klein, P. J., & Meltzoff, A. N. (1999). Long-term memory, forgetting, and deferred imitation in 12-month-old infants. Developmental Science, 2(1), 102-113.
Lenneberg, E. (1967). Biological foundations of language. New York, NY: John Wiley & Sons.
Lewis, T. L., Maurer, D., & Milewski, A. (1979). The development of nasal detection in young infants. Investigative Ophthalmology and Visual Science Supplement, 19, 271.
Li, Y., & Ding, Y. (2017). Human visual development. In Y. Liu., & W. Chen (Eds.), Pediatric lens diseases (pp. 11-20). Singapore: Springer.
Mandel, D. R., Jusczyk, P. W., & Pisoni, D. B. (1995). Infants’ recognition of the sound patterns of their own names. Psychological Science, 6(5), 314–317.
Moeller, M.P., & Tomblin, J.B. (2015). An introduction to the outcomes of children with hearing loss study. Ear and Hearing, 36 Suppl (0-1), 4S-13S.
Penfield, W., & Roberts, L. (1959). Speech and brain mechanisms. Princeton, NJ: Princeton University Press.
Petitto, L. A., & Marentette, P. F. (1991). Babbling in the manual mode: Evidence for the ontogeny of language. Science, 251(5000), 1493–1496.
Phelps, B. J. (2005). Habituation. In N. J. Salkind (Ed.), Encyclopedia of human development (pp. 597-600). New York: Sage Publications.
Piaget, J. (1954). The construction of reality in the child. New York: Basic Books.
Pickens, J., Field, T., Nawrocki, T., Martinez, A., Soutullo, D., & Gonzalez, J. (1994). Full-term and preterm infants’ perception of face-voice synchrony. Infant Behavior and Development, 17(4), 447-455.
Porter, R. H., Makin, J. W., Davis, L. M., Christensen, K. (1992). Responsiveness of infants to olfactory cues from lactating females. Infant Behavior and Development, 15, 85-93.
Rovee-Collier, C. (1987). Learning and memory in infancy. In J. D. Osofsky (Ed.), Handbook of infant development, (2nd, ed., pp. 98-148). New York: Wiley.
Rovee-Collier, C. (1990). The “memory system” of prelinguistic infants. Annuals of the New York Academy of Sciences, 608, 517-542. doi: 10.1111/j.1749-66231990.tb48908.
Rovee-Collier, C., & Hayne, H. (1987). Reactivation of infant memory: Implications for cognitive development. In H. W. Reese (Ed.), Advances in child development and behavior. (Vol. 20, pp. 185-238). London, UK: Academic Press.
Rymer, R. (1993). Genie: A scientific tragedy. Harmondsworth: Penguin.
Sen, M. G., Yonas, A., & Knill, D. C. (2001). Development of infants’ sensitivity to surface contour information for spatial layout. Perception, 30, 167-176.
Senghas, R. J., Senghas, A., & Pyers, J. E. (2005). The emergence of Nicaraguan Sign Language: Questions of development, acquisition, and evolution. In S. T. Parker, J. Langer, & C. Milbrath (Eds.), Biology and knowledge revisited: From neurogenesis to psychogenesis (pp. 287–306). Mahwah, NJ: Lawrence Erlbaum Associates.
Shaffer, D. R. (1985). Developmental psychology: Theory, research, and applications. Belmont, CA: Wadsworth, Inc.
Skinner, B. F. (1953). Science and human behavior. NY: Free Press.
Spelke, E. S., & Cortelyou, A. (1981). Perceptual aspects of social knowing: Looking and listening in infancy. Infant social cognition, 61-84.
Stika, C.J., Eisenberg, L.S., Johnson, K.C. Henning, S.C., Colson, B.G., Ganguly, D.H., & DesJardin, J.L. (2015). Developmental outcomes of early-identified children who are hard of hearing at 12 to 18 months of age. Early Human Development, 9(1), 47-55.
Thomas, R. M. (1979). Comparing theories of child development. Santa Barbara, CA: Wadsworth.
Tomblin, J. B., Harrison, M., Ambrose, S. E., Walker, E. A., Oleson, J. J., & Moeller, M. P. (2015). Language outcomes in young children with mild to severe hearing loss. Ear and hearing, 36 Suppl 1(0 1), 76S–91S.
Werker, J. F., Pegg, J. E., & McLeod, P. J. (1994). A cross-language investigation of infant preference for infant-directed communication. Infant Behavior and Development, 17, 323-333.
Werker, J. F., & Tees, R. C. (2002). Cross-language speech perception: Evidence for perceptual reorganization during the first year of life. Infant Behavior and Development, 25, 121-133.
United States National Library of Medicine. (2016). Circumcision. Retrieved from https://medlineplus.gov/circumcision.html
OER Attribution: “Lifespan Development: A Psychological Perspective, Second Edition” by Martha Lally and Suzanne Valentine-French is licensed under a CC-BY-NC-SA-3.0
Media Attributions
- palmergrasp © jelly is licensed under a CC0 (Creative Commons Zero) license
- alireza-attari-mp_FNJYcjBM-unsplash © Alireza Attari is licensed under a CC0 (Creative Commons Zero) license
- 640px-Baby_Face © Avsar Aras is licensed under a CC BY-SA (Attribution ShareAlike) license
- assimilation © Lally & Valentine-French is licensed under a CC BY-NC-SA (Attribution NonCommercial ShareAlike) license
- tai-s-captures-y4cV-gQqmVI-unsplash-2 © Tai's Captures is licensed under a CC0 (Creative Commons Zero) license
- stage6
- Baillargeon © Lally & Valentine-French is licensed under a CC BY-NC-SA (Attribution NonCommercial ShareAlike) license
- 640px-Human-Male-White-Newborn-Baby-Crying © Evan-Amos is licensed under a Public Domain license
- Noam_Chomsky_ © Augusto Starita is licensed under a CC BY-SA (Attribution ShareAlike) license
- brocasarea © Lally & Valentine-French is licensed under a CC BY-NC-SA (Attribution NonCommercial ShareAlike) license
- VictorofAveyron © Unknown is licensed under a Public Domain license
- B.F._Skinner_at_Harvard_circa_1950 © Silly rabbit is licensed under a CC BY (Attribution) license
- 339px-Albert_Bandura_Psychologist © Albert Bandura is licensed under a CC BY-SA (Attribution ShareAlike) license

